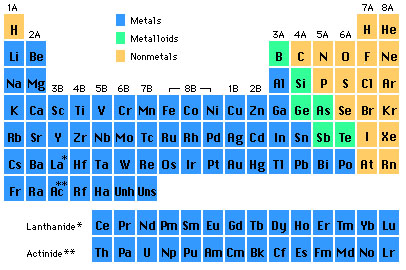
Elements in Group 2a Are Known as the
1and 2 group elements are generally have large atomic size so they easily lose electrons and forms positive ions Ie they can easily oxidise and gives electrons easily. For example in group 2A elements beryllium is the smallest in size whereas radium being at the bottom is the largest in size.

Alkaline Earths Group 2a Elements Definition Properties Video Lesson Transcript Study Com
Groups IIA IIIA and IVA.

. Alkaline earth metals include beryllium Be magnesium Mg calcium. They have low electron affinity. Answer to The elements in Group 2A are known by what name.
These elements also known as alkaline earth metals are. The elements in Group IIA Be Mg Ca Sr Ba and Ra are all metals and all but Be and Mg are active metals. They are sometimes also called the representative elements and they are the most abundant in the universe.
Stands two s subshell -electrons. Beryllium Be Magnesium Mg. They have low electronegativity.
The f-block elements in period 7 are known as the. Elements in group 7A are known as. The elements in Group 2A are known by what name.
Group 1 metals are less reactive than group 2 metals. One such group of elements is in the second column of the periodic table and is known as group 2 elements. They are harder and less reactive than the alkali metals of Group 1A.
They share many characteristics. A transition metals B halogens C alkali metals D alkaline earth metals E noble gases. Alkali metals O noble gases O transition metals alkaline earth metals O halogens How many core electrons does a SolutionInn.
The elements in group 2a are known by what name a. QUESTION 43 The elements in Group 2A are known by what name. The periodic table also known as the periodic table of the chemical elements is a tabular display of the chemical elementsIt is widely used in chemistry physics and other sciences and is generally seen as an icon of chemistryIt is a graphic formulation of the periodic law which states that the properties of the chemical elements exhibit a periodic dependence on their atomic.
See answer 1 Best Answer. The elements in Group 2A are known by what name. The name comes from the fact that when these metals or their oxides are dissolved in water a basic alkaline.
Beryllium Be magnesium Mg calcium Ca strontium Sr barium Ba and radium Ra. The name comes from the fact that the oxides of these metals produced basic solutions when dissolved in water and they remained solids at the. Chemistry questions and answers.
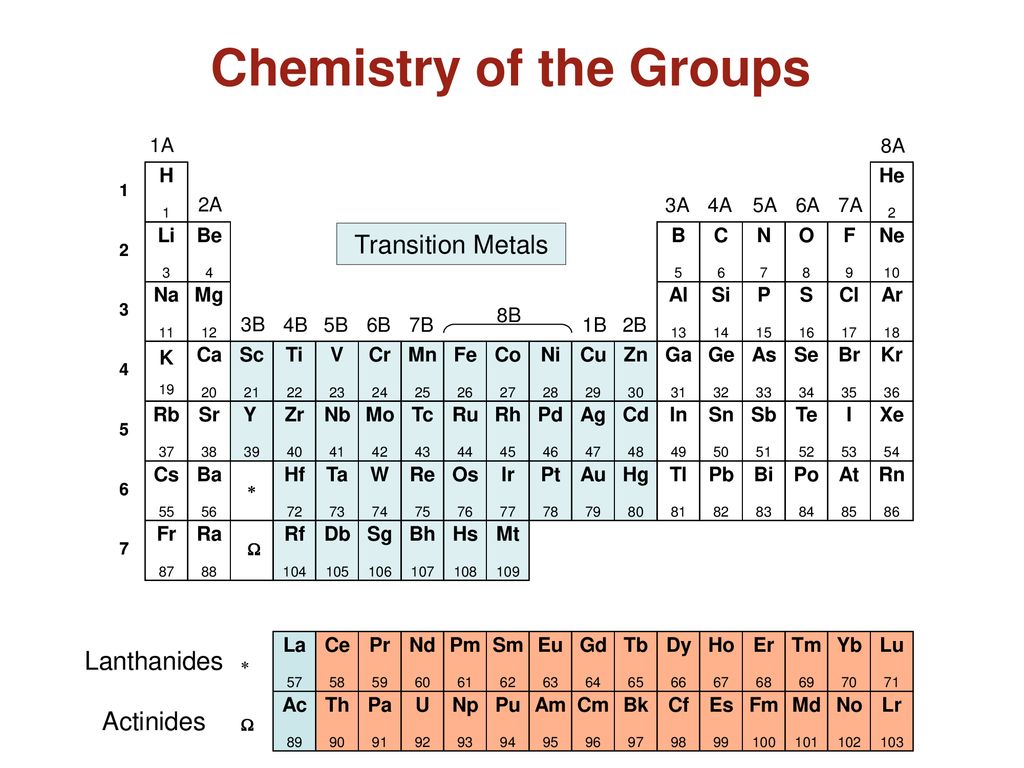
Answer 1 of 2. This is the manganese group. Mn Tc Re Bh.
1 A alkaline earth metals. Also atomic number of beryllium is 4 and atomic number of radium is 88. Its valence shell contains 2 electrons.
Group 2A or IIA of the periodic table are the alkaline earth metals. Thus we can conclude that out of the given options radium is the 2A element which has the largest atomic radius. Answer 1 of 3.
The term earth was historically used to describe the. The d-block elements are known as. What elements are found in Group 2A.
Alkaline earth metals alkali metals noble gases O halogens transition metals QUESTION 44 Which statement concerning atoms is FALSE. Beryllium magnesium calcium strontium barium and radium arethe group IIA elements. Stands for one p subshell -electron.
The elements in group 2A are called. These elements are often called the alkaline-earth metals. 41 Elements in Group 2A are known as the A alkaline earth metals B alkali metals C chalcogens D halogens E noble gases 42 Elements in Group 7A are known as the A chalcogens B alkali metals C alkaline earth metals D halogens E noble.
How many valence electrons does group 6A elements have compared to group. N -stands for n th shell. The elements of the periodic table in groups 1A to 8A are called the main-group elements.
So they can reduse gaining electronsother atoms strongly. Some of the main group elements have common names such as the alkali metals 1A the alkaline earths 2A the halogens 7A and the noble gases 8A. They are metal due to 1luster shine 2good conductors of heat and electricity 3typically solid at room temperature 4most are malleable and ductile 5.
Which one of these elements is a transition element. Sometimes group 2A is represented by roman numerals or IIA. Alkaline earth metals E.
Group 1A or IA of the periodic table are the alkali metals. Soacts as best reducing agents. This is the manganese group.
The term alkaline reflects the fact that many compounds of these metals are basic or alkaline. Chemistry questions and answers. Electrons reside outside the nucleus in what is called the electron cloud The atomic number of an atom is the number of.
Group 2 elements are called alkaline metals because they form alkaline solutions hydroxides when reacting with water and their oxides are found in the earths crust. Metals include the areas shaded light blue and include the families 1A 2A. Hydrogen H lithium Li sodium Na potassium K rubidium Rb cesium Cs and francium FrThese are except for hydrogen soft shiny low-melting highly reactive metals which tarnish when exposed to air.
The elements in group 1A except hydrogen are called. A alkaline earth metals B alkali metals C chalcogens D halogens E noble gases Answer. 15 Elements in Group 2A are known as the _____.
The alkali metal elements are found in _____ of the periodic table.
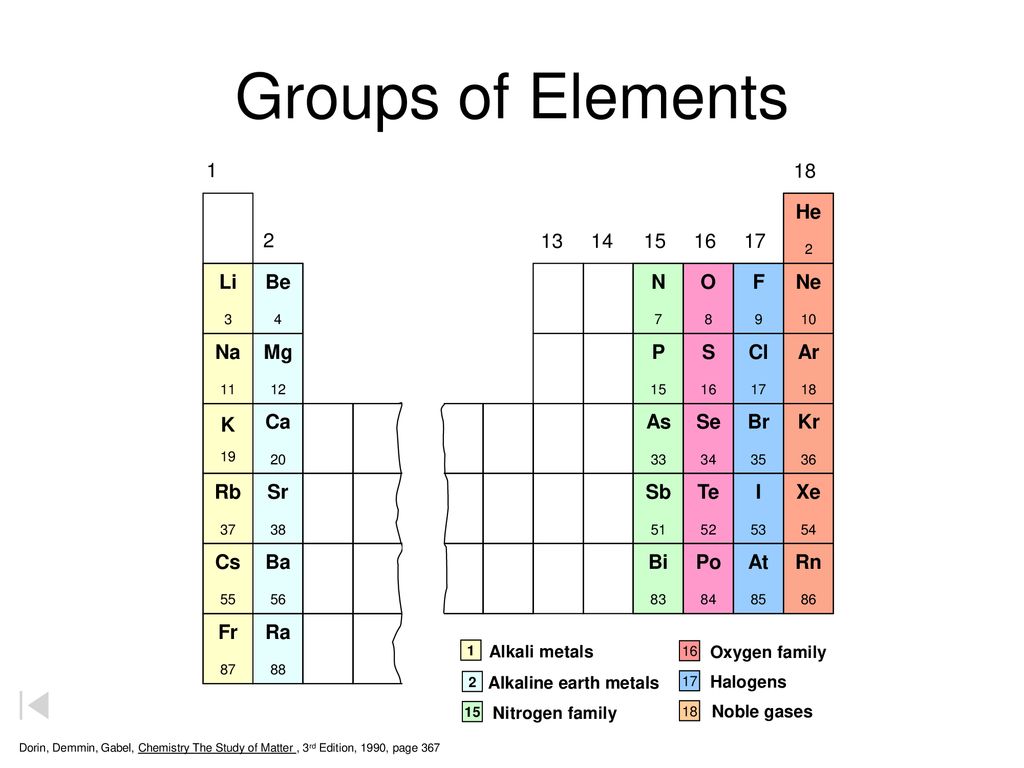
Groups Of Elements 1a 8a H He 2a 3a 4a 5a 6a 7a Li Be B C N O F Ne Na Ppt Download
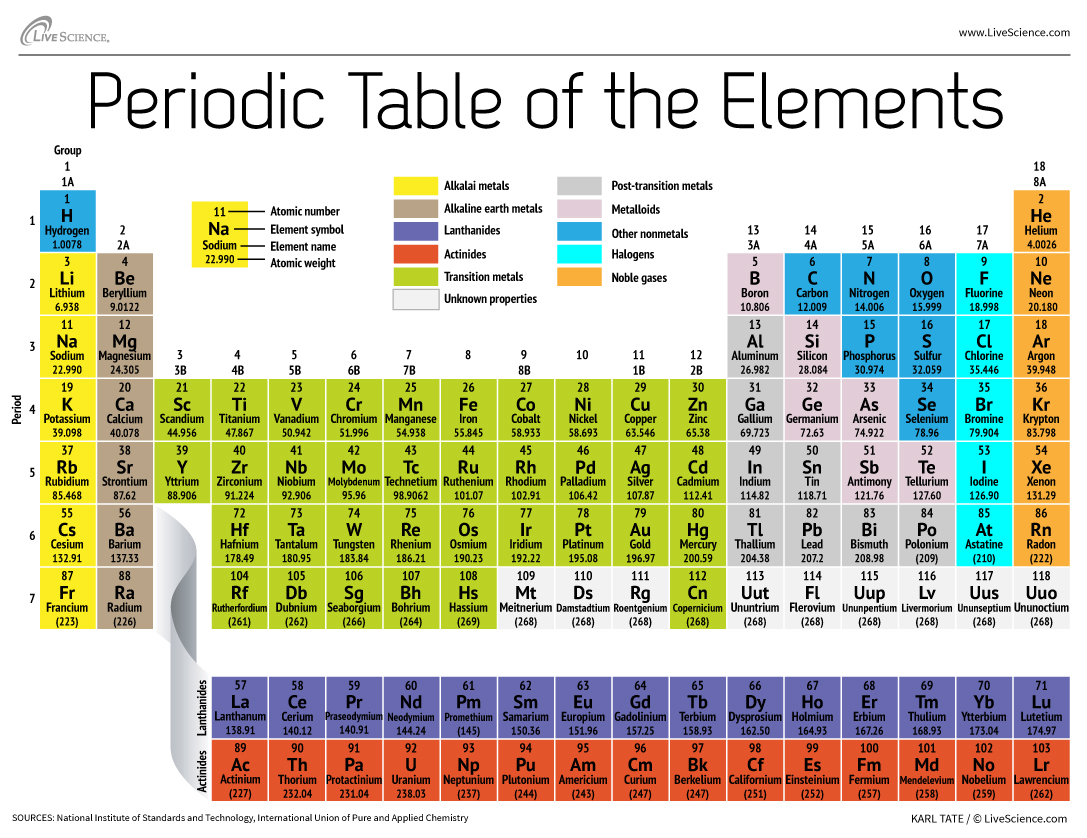
How The Periodic Table Groups The Elements Live Science
Groups Of Elements 1a 8a H He 2a 3a 4a 5a 6a 7a Li Be B C N O F Ne Na Ppt Download
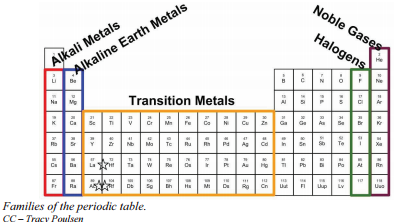
2 3 Families And Periods Of The Periodic Table Chemistry Libretexts
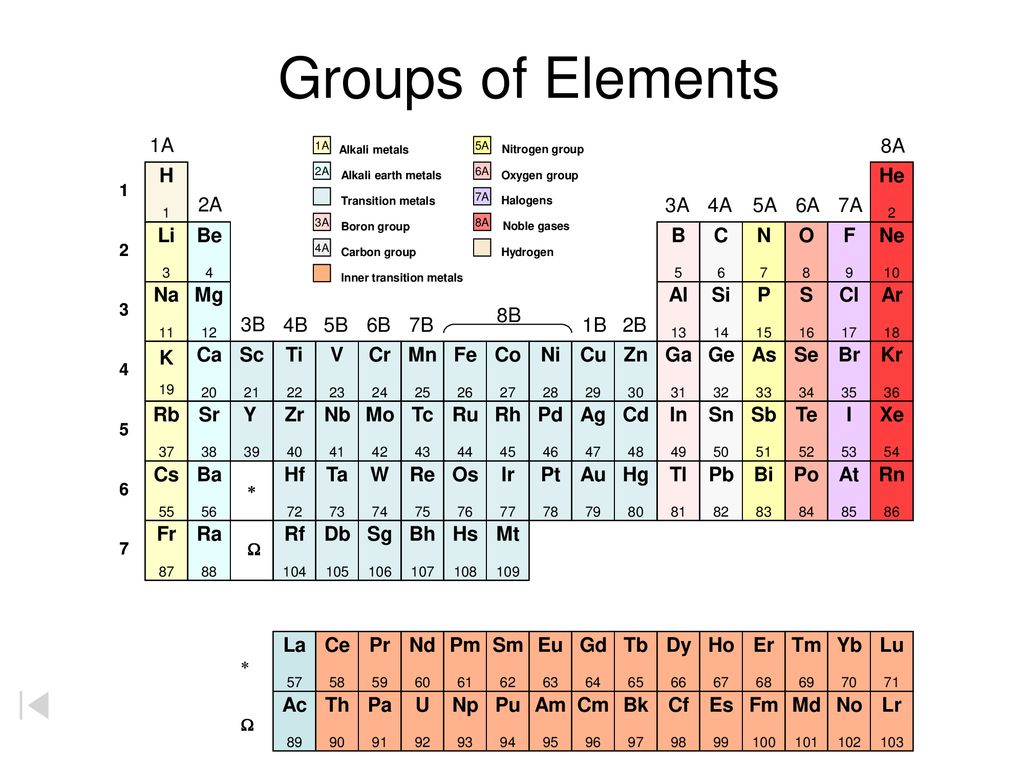
Groups Of Elements 1a 8a H He 2a 3a 4a 5a 6a 7a Li Be B C N O F Ne Na Ppt Download
What S The Name Of The Columns In The Periodic Table Quora

Groups Of Elements 1a 8a H He 2a 3a 4a 5a 6a 7a Li Be B C N O F Ne Na Ppt Download

Alkali Earth Metals Group 2 Elements Properties Characteristics Videos
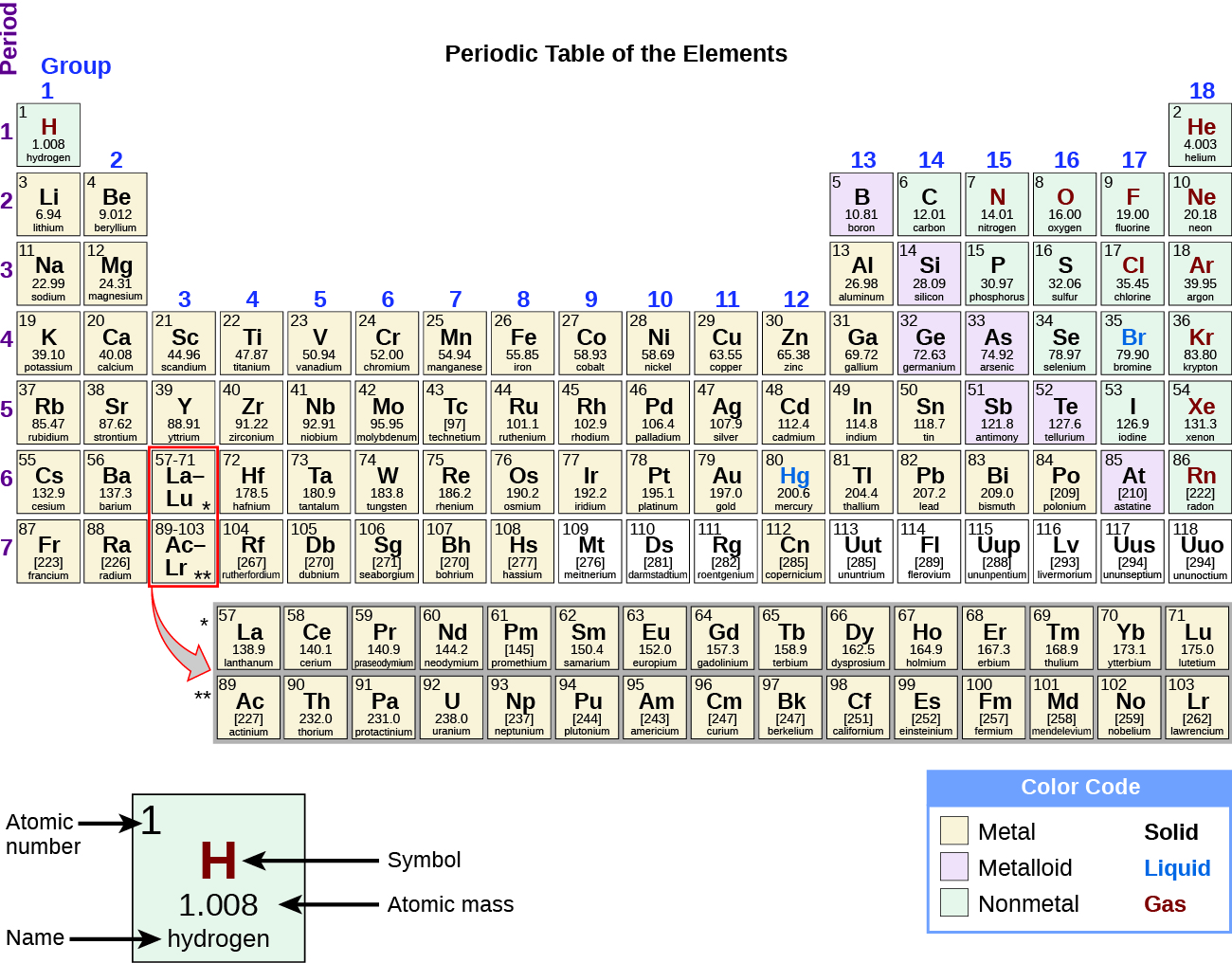
2 5 The Periodic Table Chemistry

Periodic Table Alkali Metals Group 1a Alkaline Metals Group 2a Transition Metals Group B Metalloids 7 Purple Ele Periodic Table Transition Metal Alkali Metal

Representative Elements Of The Periodic Table Video Lesson Transcript Study Com

Alkaline Earth Metal Properties List Reactivity Britannica

Group 2 Elements Alkaline Earth Metals Emedicalprep

Term 2 Sqp Choose An Element From Period 3 Of Modern Periodic Table

Group 2 Elements Alkaline Earth Metals Emedicalprep

Representative Elements Of The Periodic Table Video Lesson Transcript Study Com